Interactions with Pol II
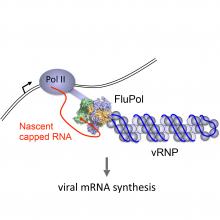
Influenza viruses hijack the transcriptional machinery of their host to synthesize their own viral mRNAs. Specifically, the viral RNA polymerase binds to the C-terminal domain (CTD) of the large subunit of host RNA polymerase II (Pol II). This close association with the host transcriptional machinery allows the viral RNA polymerase to access nascent host transcripts. By a process known as cap-snatching, the viral RNA polymerase steals short 5' capped RNA fragments from host capped RNAs and uses them as primers for transcribing viral mRNAs (Walker and Fodor 2019).
We showed that the influenza virus RNA polymerase interacts with host Pol II and the interaction was mediated by the serine-5 phosphorylated CTD (Engelhardt et al 2005; Martínez-Alonso et al 2016). In collaboration with Jonathan Grimes’ group we solved the structure of the influenza C virus polymerase bound to a CTD mimic peptide and proposed that binding to the CTD stabilises the viral RNA polymerase in a transcriptase conformation, activating its cap-snatching activity (Serna Martin et al 2018). As a result of cap-snatching, host gene expression is inhibited, contributing to the virus induced host shut off and the inhibition of antiviral host responses (Bauer et al 2018).